
Why does atomic radius decrease as electrons are added?Ītomic radius decreases across a period because valence electrons are being added to the same energy level at the same time the nucleus is increasing in protons. The loss in an electron will consequently result in a change in atomic radii in comparison to the neutral atom of interest (no charge). Does losing electrons increase atomic radius? The increase in nuclear charge attracts the electrons more strongly, pulling them closer to the nucleus. What is atomic radius Why does atomic radius decreases across a period?Ītomic radius decreases across a period because valance electrons are being added to the same energy level at the same time the nucleus is increasing in protons. So Cesium has the largest atomic radius among the given elements. increases The ionic size tends to _ down a group.Which of the following has the largest atomic radius? So it is more difficult to remove an electron from a nonmetal than an alkali metal The atomic radius _ down a group. The nuclear charge increases from left to right across a period and the shielding effect stays the same. Nonmetals have smaller atoms, while metals have larger atoms and therefore less attraction of the electrons to the nucleus. Why is the first ionization energy of a nonmetal much higher than that of an alkali metal? -Nonmetals have more filled shells, making it harder to remove electrons.

Across a period, you add electrons and protons, and so the electronegativity increases but the atomic radius decreases. What did Mendeleev base his arrangement of the periodic table on? Average atomic mass Why does atomic size generally increase as you move down a group of the periodic table and decrease as you move from left to right across a period? As you go from top to bottom, your increasing 1 energy level and the shielding affect comes into place, allowing atomic size to increase. What are the trends for ionization energy first ionization tends to decrease from top to bottom within a group and increase from left to right across a period Why was it important for scientists to find a logical way to organize the elements? It made it possible for scientists to determine how many elements they were looking for. When are positive and negative ions formed? When an electron is added or taken away from the atom. For representative elements, the values tend to increase from left to right across a period. decrease What are the trends for electronegativity Electronegativity values decrease from to bottom within a group.

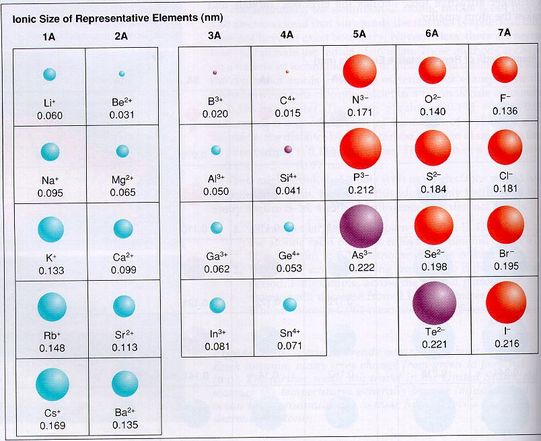
decreases What type of element tends to form anions? Nonmetals Ionic size tends to _ across a period.
#Atomic radius increases down a group series#
Inner Transition Metal an element in the lanthanide or actinide series the highest occupied s sublevel and nearby f sublevel of its atoms generally contain electrons also called inner transition element Atomic Radius one half the distance between the nuclei of two atoms of the same element when they are joined Ion an atom or group of atoms that has a positive or negative charge Cation any atom or group of atoms with a positive charge Anion any atom or group of atoms with a negative charge Ionization Energy the energy required to remove an electron from an atom in its gaseous state Electronegativity the ability of an atom to attract electrons when the atom is in a compound The atomic radius _ across a period.


 0 kommentar(er)
0 kommentar(er)
